Effect of interionic interactions on the structure and dynamics of ionic solvation shells in aqueous electrolyte solutions†
Abstract
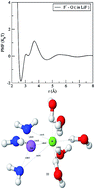
We have performed molecular dynamics (MD) simulations to explore the structure and dynamics of the ionic solvation shells of alkali ions and halide ions in aqueous solution. Several structural and dynamical properties such as radial distribution functions (RDFs), diffusivity, velocity autocorrelation function (VACF), etc. are computed to obtain a microscopic picture of solvation. In addition, we have examined the effect of counter ions on the solvation structure of ions. We have also calculated the mean residence time (MRT) of solvent molecules in the first solvation shell (FSS) using a stable states picture (SSP) approach. The results from this work can provide a microscopic picture of the underlying mechanisms which play an important role in determining macroscopic properties in electrolyte solutions.

 Please wait while we load your content...
Please wait while we load your content...