Effects of Li2SO4·H2O amounts on morphologies of hydrothermal synthesized LiMnPO4 cathodes
Abstract
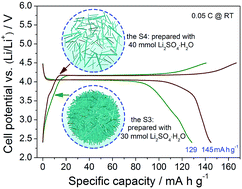
Developing an industrial LiMnPO4 cathode, featuring a high rate capability, is still a huge challenge because of its slow Li+ diffusion speed as well as low electronic conductivity. Here, we expect to address these inherent obstacles by controlling its morphology in a well-dispersed way. The effects of the added amounts of Li2SO4·H2O on the morphologies of the LiMnPO4 samples prepared via the hydrothermal method were studied using structural and morphological characterizations. Based on the additional independent experiments, a two-step reaction was tentatively proposed to understand the formation of the LiMnPO4 samples and the dual functions of Li2SO4·H2O. For synthesizing LiMnPO4 samples, a high SO42− concentration may be helpful for a fast nucleation rate, while a high Li+ concentration can be beneficial for a rapid growth rate. The results showed that the LiMnPO4 sample prepared with 40 mmol Li2SO4·H2O (denoted as the S4) exhibited the best electrochemical performances in terms of the discharge capacity, the rate capability and the cycling stability among all the LiMnPO4 samples, which was reasonably attributed to its well-dispersed characteristics.

 Please wait while we load your content...
Please wait while we load your content...