Improved preparation of 4(5)-aryl-2-(β-d-glucopyranosyl)-imidazoles, the most efficient glucose analogue inhibitors of glycogen phosphorylase†
Abstract
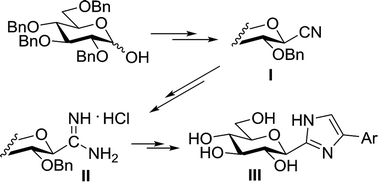
The synthesis of 4(5)-aryl-2-(β-D-glucopyranosyl)-imidazoles, the currently most efficient glucose derived inhibitors of glycogen phosphorylase enzymes was amended and extended by using O-perbenzylated β-D-glucopyranosyl cyanide as the starting material. This compound and its derivatives C-(β-D-glucopyranosyl)formimidate and formamidine were obtained in large scale reactions to give the products in ∼20 grams amounts. Ring closing reactions of the formimidate and formamidine by α-amino- and α-bromoketones, respectively, produced the O-perbenzylated imidazoles which were deprotected by catalytic hydrogenation or by EtSH/BF3·OEt2. Newly prepared 4(5)-(4-nitro- and -aminophenyl)-2-(β-D-glucopyranosyl)-imidazoles proved less efficient inhibitors (Ki values of 1141 and 411 nM, respectively) than their unsubstituted counterpart (Ki = 280 nM).


 Please wait while we load your content...
Please wait while we load your content...