Guest-induced stereoselective self-assembly of quinoline-containing PdII and PtII metallacycles†
Abstract
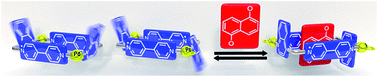
A family of mono- and dinuclear metallacycles has been self-assembled from (en)M(NO3)2 metal centers (M = PdII or PtII) and 4,4′-bipyridinium/2,7-diazapyrenium-based ligands incorporating quinoline subunits in their structures. These receptors recognize aromatic substrates, up to the dimensions of naphthalene, by means of hydrophobic forces, π–π stacking and C–H⋯π interactions. In the case of the M2L2 receptors, the quinoline moieties in the short sides of these molecular rectangles, cause the existence of two atropisomers with different cavity characteristics. The formation of inclusion complexes, derived from the dinuclear hosts and appropriate aromatics, induced stereoselectivity on the self-assembly, which was found to be modulated mainly by the size of the guests and their ability to optimize the number of potential C–H⋯π interactions with the two isomeric receptors. In addition, the π-acceptor and hydrophobic character of the host also contributes to this stereoselectivity. The proposed mechanism for the interconversion of the syn/anti isomers, studied by DFT methods, involves the rotation of the quinoline rings without dissociation of the self-assembled metallacycles.

 Please wait while we load your content...
Please wait while we load your content...