Following the thermal and chemical activation of supported Au clusters using X-ray absorption spectroscopy†
Abstract
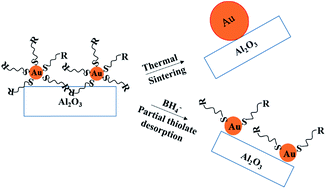
Al2O3-supported Au25(SC8H9)18 clusters with various Au loadings were thermally and chemically treated in order to determine the most efficient method towards the removal of thiolate stabilizers while avoiding unwanted increases in cluster size due to agglomeration and sintering. X-ray absorption spectroscopy (XAS) and transmission electron microscopy (TEM) were used to investigate samples before and after thermal and chemical treatments. Results show that while 250 °C thermal treatment leads to nearly complete removal of thiolate stabilizers, it comes with a concomitant increase in cluster size as sintering becomes problematic. In contrast, chemical reduction treatments using borohydride reducing agents does not lead to significant growth in cluster size, but only allows for partial thiolate removal. These results are important as many researchers look to determine optimal activation conditions for ultra-monodisperse Au and bimetallic clusters for use as model catalysts without destroying their original structures.

 Please wait while we load your content...
Please wait while we load your content...