Bioactive (co)oligoesters with antioxidant properties – synthesis and structural characterization at the molecular level
Abstract
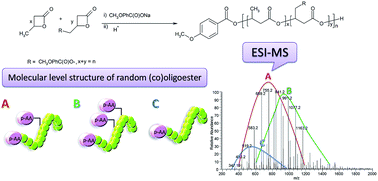
A contemporary synthetic route, starting from the bioactive compound via the corresponding glycidyl ester and β-substituted β-lactone to (homo)- and (co)oligoesters with a bioactive moiety covalently linked as pendent groups along an oligomer backbone, was reported. The bioactive compounds were selected from antioxidants used in cosmetics. Two models of bioactive (homo)- and (co)oligoesters were synthesized via anionic ring-opening (co)oligomerization of p-methoxybenzoyloxymethylpropiolactone (p-AA-CH2-PL) initiated by p-anisic acid sodium salt. An analytical protocol was developed for a detailed structural characterization at the molecular level of these bioactive (co)oligoesters. The molecular level structure of the obtained bioactive (homo)- and (co)oligoesters was established by electrospray ionization tandem mass spectrometry (ESI-MS/MS) supported by 1H NMR analysis. Additionally, the results presented here are important for the analysis of designed biodegradable polymeric controlled-release systems of bioactive compounds with potential applications in cosmetology.

 Please wait while we load your content...
Please wait while we load your content...