Rhodium-catalysed tandem dehydrogenative coupling–Michael addition: direct synthesis of phthalides from benzoic acids and alkenes†
Abstract
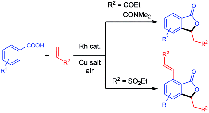
[(COD)RhCl]2 catalyses the coupling of benzoic acids and alkenes in the presence of Cu(OAc)2·H2O and dicyclopentadiene to afford phthalides in 15–93% yield. A 3-substituted or 3,7-disubstituted product is obtained selectively depending on alkene type. The reaction is highly atom-economical and uses readily available starting materials.

 Please wait while we load your content...
Please wait while we load your content...