H2 activation using the first 1 : 1 : 1 hetero-tri(aryl)borane†
Abstract
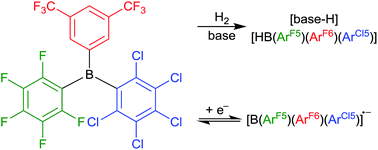
The novel 1 : 1 : 1 hetero-tri(aryl)borane (pentafluorophenyl){3,5-bis(trifluoromethyl)phenyl}(pentachlorophenyl)borane has been synthesised and structurally characterised. This has been show to act as the Lewis acidic component in FLPs for the heterolytic cleavage of H2 with three Lewis bases.


 Please wait while we load your content...
Please wait while we load your content...