Mutual solubility of water and hydrophobic ionic liquids in the presence of hydrochloric acid†
Abstract
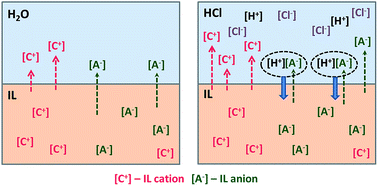
Previous studies have shown that the presence of nitric acid, the principal solute in various hydrometallurgical processes, and perchloric acid in the aqueous phase is an important factor for increased aqueous solubility of hydrophobic ionic liquids. In this study, the effect of hydrochloric acid in the aqueous phase on the mutual solubility of water and hydrophobic 1,3-dialkylimidazolium- and N,N-dialkylpyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquids, [Cnmim][Tf2N] (n = 2, 4, 6, and 8) and [C3C1pyrr][Tf2N], is examined at room temperature and atmospheric pressure. Hydrochloric acid caused a considerable increase in the aqueous solubility of all the studied ionic liquids. The amount of water transferred into the organic phase increases with increasing hydrochloric acid concentration for short alkyl chain ILs, and the opposite trend was observed for long alkyl chain ILs. The effect of the N–H acid bis(trifluoromethylsulfonyl)imide, H[Tf2N], and the salts lithium bis(trifluoromethylsulfonyl)imide, Li[Tf2N], and 1-butyl-3-methylimidazolium chloride, [C4mim]Cl, dissolved in hydrochloric acid solutions on the mutual solubility of water and the [C4mim][Tf2N] ionic liquid were also investigated. The salting-out effect is observed and it was shown to be dependent on the nature of the salt, its concentration and the hydrochloric acid concentration in the aqueous phase. A mathematical model has been developed to describe the dependence of the ionic liquid cation and anion concentration on the common ion salt concentration in the aqueous hydrochloric acid phase. This model describes the basic character of ionic liquid dissolution in the aqueous phase and allows for estimation of solubility product values.

 Please wait while we load your content...
Please wait while we load your content...