Synthesis of new α-aminophosphonate derivatives incorporating benzimidazole, theophylline and adenine nucleobases using l-cysteine functionalized magnetic nanoparticles (LCMNP) as magnetic reusable catalyst: evaluation of their anticancer properties†
Abstract
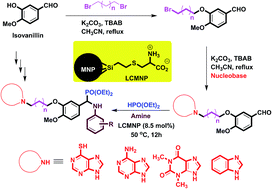
A new class of structurally diverse α-aminophosphonate derivatives containing benzimidazole, theophylline and adenine heterocycles were synthesized using a simple and efficient strategy. This class of α-aminophosphonates was synthesized using the reaction of synthetic aldehydes containing nucleobases, amines and diethyl phosphonate using L-cysteine functionalized magnetic nanoparticles (LCMNP) as catalyst. The aldehyde derivatives containing benzimidazole, theophylline and adenine nucleobases were synthesized using the reaction of a bromo-substituted aldehyde derived from isovanillin and 4-hydroxy benzaldehyde. The LCMNP catalyst was found to be an efficient magnetic reusable catalyst for synthesis of this class of α-aminophosphonates under mild and clean conditions. This method is introduced as a suitable approach for the synthesis of new α-aminophosphonate derivatives containing nucleobases which have potential biological activity. When the anticancer properties of a selected group of these synthetic ligands were evaluated, they indicated poor activity compared to cisplatin against the Jurkat cancer cell line.

 Please wait while we load your content...
Please wait while we load your content...