Regioselective α-arylation of coumarins and 2-pyridones with phenylhydrazines under transition-metal-free conditions†
Abstract
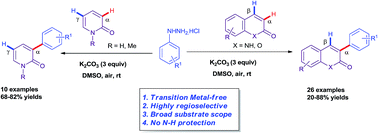
A facile regioselective metal-free direct α-arylation of coumarins and 2-pyridones is achieved by the reaction of coumarins and 2-pyridones with phenylhydrazine in good yields. The reaction proceeds at room temperature under mild conditions using inexpensive reagents and without the need for step intensive activating groups. The methodology is operationally simple, practically viable and also allows the coupling of similar nitrogen heterocycle aza-coumarins without prerequisite N-protection.

 Please wait while we load your content...
Please wait while we load your content...