Dependence on size of supported Rh nanoclusters for CO adsorption†
Abstract
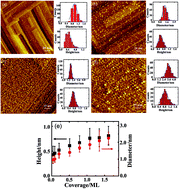
We studied the adsorption and lateral interactions of CO molecules on Rh nanoclusters supported on an ordered thin film of Al2O3/NiAl(100) with varied surface probe techniques under ultra-high vacuum conditions and with density-functional-theory (DFT) calculations. The Rh clusters were grown with vapor deposition onto the Al2O3/NiAl(100) surface at 300 K; with increasing deposition, their mean diameter evolved from 1.0 to 3.5 nm and their height from 0.4 to 0.8 nm. The initial adsorption energy (for sparse CO coverage) and the number of adsorbed CO per surface site on the Rh clusters increased with decreasing cluster size. The former effect results from the surface structure and expanded lattice parameter of small Rh clusters, whereas the latter effect involves not only the initial adsorption energy but also altered lateral interactions among CO molecules. In contrast with CO on Rh single crystals, CO on small clusters adsorbed with their axes tilted from the surface normal, weakening the CO–CO repulsive interactions for CO coverage over a wide range. The saturated density of CO on clusters of diameter near 1.0 nm and height near 0.4 nm is 2–3 times that of large clusters (diameter ≥ 3.5 nm) or a Rh(100) surface. The CO–CO repulsive interactions on small clusters became effective at large CO densities, given that the onset of desorption of CO at saturation was 100 K lower than that of large clusters.

 Please wait while we load your content...
Please wait while we load your content...