Acetone as a polar cosolvent for pyridinium-based ionic liquids†
Abstract
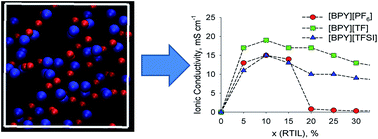
This work reports transport and structure properties of the three pyridinium-based ionic liquids (RTILs) – N-butylpyridinium hexafluorophosphate [BPY][PF6], N-butylpyridinium trifluoromethanesulfonate [BPY][TF] and N-butylpyridinium bis(trifluoromethanesulfonyl)imide [BPY][TFSI] – in their mixtures with acetone (ACET). The ionic conductivity maximum occurs at 10 mol% RTIL, irrespective of the anion. The absolute ionic conductivity value of [BPY][TF] is higher than those of other RTILs. This is explained by a weaker cation-anion binding than that in [BPY][PF6] and smaller anion size than that of [BPY][TFSI]. All the investigated RTILs are infinitely miscible with ACET, which boosts their diffusivity and conductivity. A small viscosity of ACET favors a drastic viscosity decay in the RTIL–ACET mixtures. Structure analysis (radial distribution functions, ionic clusters) of the mixtures are in line with their transport properties providing a reliable microscopic interpretation of the observed macroscopic properties. As a molecular co-solvent, ACET constitutes an interesting alternative and competitor to more intensively investigated liquids.

 Please wait while we load your content...
Please wait while we load your content...