Using a biomimetic membrane surface experiment to investigate the activity of the magnetite biomineralisation protein Mms6†
Abstract
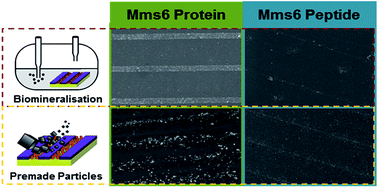
Magnetotactic bacteria are able to synthesise precise nanoparticles of the iron oxide magnetite within their cells. These particles are formed in dedicated organelles termed magnetosomes. These lipid membrane compartments use a range of biomineralisation proteins to nucleate and regulate the magnetite crystallisation process. A key component is the membrane protein Mms6, which binds to iron ions and helps to control the formation of the inorganic core. We have previously used Mms6 on gold surfaces patterned with a self-assembled monolayer to successfully produce arrays of magnetic nanoparticles. Here we use this surface system as a mimic of the interior face of the magnetosome membrane to study differences between intact Mms6 and the acid-rich C-terminal peptide subregion of the Mms6 protein. When immobilised on surfaces, the peptide is unable to reproduce the particle size or homogeneity control exhibited by the full Mms6 protein in our experimental setup. Moreover, the peptide is unable to support anchoring of a dense array of nanoparticles to the surface. This system also allows us to deconvolute particle binding from particle nucleation, and shows that Mms6 particle binding is less efficient when supplied with preformed magnetite nanoparticles when compared to particles precipitated from solution in the presence of the surface immobilised Mms6. This suggests that Mms6 binds to iron ions rather than to magnetite surfaces in our system, and is perhaps a nucleating agent rather than a controller of magnetite crystal growth. The comparison between the peptide and the protein under identical experimental conditions indicates that the full length sequence is required to support the full function of Mms6 on surfaces.

 Please wait while we load your content...
Please wait while we load your content...