A concise total synthesis of sespenine, a structurally unusual indole terpenoid from Streptomyces†
Abstract
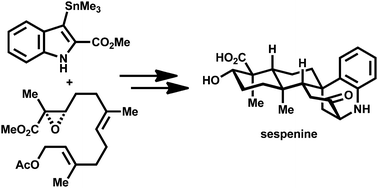
Sespenine is a structurally unusual indole sesquiterpenoid isolated from endophytic Streptomyces sp. HKI0595. Herein, we report a ten-step (the longest linear sequence) synthesis of this molecule from commercially available materials, on the basis of our first generation synthesis. Sharpless asymmetric epoxidation and Stille–Miyata coupling were used to construct a functionalized epoxy ester, which underwent Ti(III) mediated reductive radical cyclization to give a trans-decalin intermediate with a 2-methoxycarbonylindole side chain. Oxidation of this compound afforded a pair of epimeric 3-hydroxyindolenines, and the major isomer entered a bioinspired cascade of Prins cyclization/Friedel–Crafts/retro Friedel–Crafts under acidic conditions, to furnish the polycyclic core of sespenine. Sespenine analogues bearing different C2 substituents were prepared with similar chemistry. Xiamycin A, a carbazole congener of sespenine, was synthesized from the minor hydroxyindolenine epimer as well.
- This article is part of the themed collections: Celebrating 70 Years of Shanghai Institute of Organic Chemistry and 2015 Emerging Investigators by OCF

 Please wait while we load your content...
Please wait while we load your content...