Reactivity and mechanistic insight into the cross coupling reaction between isochromans and β-keto esters through C–H bond activation under visible light irradiation†
Abstract
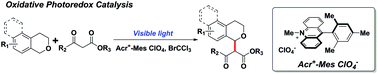
The visible light catalytic cross coupling reaction from two different C–H bonds provides an efficient protocol for C–H bond activation and C–C bond construction. The application of the oxidative photoredox strategy to couple C–H bonds next to an oxygen atom with other C–H bonds is more challenging because of the more positive oxidation potential of ethers than that of amines. Here, we take advantage of organic dye 9-mesityl-10-methylacridinium perchlorate (Acr+-Mes ClO4−) as the photosensitizer and BrCCl3 as the terminal oxidant to achieve the addition of β-keto esters to oxonium species, directly generated from isochromans, leading to the formation of alkylation products in the α-position of an oxygen atom under visible light irradiation.

 Please wait while we load your content...
Please wait while we load your content...