Regiospecific synthesis of distally chlorinated ketones via C–C bond cleavage of cycloalkanols†
Abstract
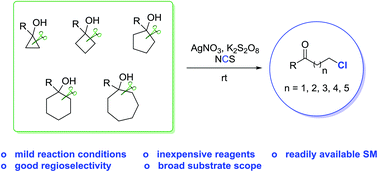
A variety of distally Csp3-chlorinated ketones are synthesized with good regioselectivities via silver-catalyzed ring opening of cycloalkanols under mild reaction conditions. The reaction uses only routine, inexpensive reagents and can be scaled up to gram quantities. A novel radical-mediated C–C bond cleavage/C–Cl bond formation is proposed.

 Please wait while we load your content...
Please wait while we load your content...