Molecular photo-charge-separators enabling single-pigment-driven multi-electron transfer and storage leading to H2 evolution from water†
Abstract
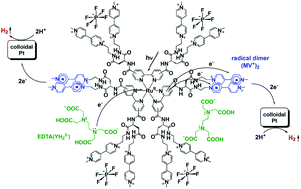
Single-chromophore-driven single-electron-pumping processes leading to multi-electron transfer and storage are effectively promoted in natural photosynthesis, generating photocurrent at the molecular level. Moreover, these single-electron-pumping events are converted into double-electron-pumping events by releasing multiple plastoquinol molecules without releasing reactive semiquinone radicals, thereby enabling storage of two-electron-reduced molecules within the lipid bilayer constructing the thylakoid membrane. Here we report new unimolecular architectures that enable these highly sophisticated light-driven multi-electron transfer and storage processes. The photo-charge-separators (PCSs) reported herein possess a single Ru(bpy)32+ fragment with each bpy derivatized with four dicationic viologen acceptors, abbreviated as [RuII(bpy)32+–(MV2+)12]26+ (MV2+ is a viologen unit). These highly positively charged PCSs form stable ion pairs with anionic electron donors, enabling consecutive multi-electron transfer processes from the donors to the pendant viologen acceptors. The multiple transferred electrons are collected over twelve pendant viologen acceptors, leading to storage of 7–8 electrons per molecule. The resulting organic radicals show a strong preference to form diamagnetic π-dimers, which suppress reactive radical formation. These reducing equivalents can then be efficiently consumed in catalytic H2 evolution from water in the presence of a colloidal platinum catalyst.

 Please wait while we load your content...
Please wait while we load your content...