Kinetic and mechanistic aspects of the iodine transfer copolymerization of vinylidene fluoride with 2,3,3,3-tetrafluoro-1-propene and functionalization into ω-hydroxy fluorinated copolymers†
Abstract
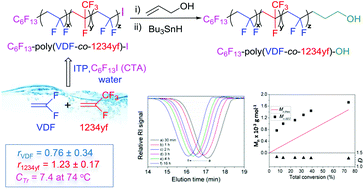
The synthesis of functional poly(VDF-co-1234yf) copolymers bearing –OH end groups was achieved via iodine transfer copolymerizations of vinylidene fluoride (VDF) with 2,3,3,3-tetrafluoro-1-propene (1234yf) followed by selective post-functionalization. First, free radical copolymerization (FRP) (in the absence of a chain transfer agent (CTA)) of VDF with 1234yf was investigated under different experimental conditions: varying the comonomer feed ([VDF]0/[1234yf]0) ratio led to several poly(VDF-co-1234yf) copolymers with molecular weights (Mn) ranging between 4600 and 12 400 g mol−1, dispersities (Đ) of ca. 2.05, and fair to good conversions (45–77%). Thermoplastic crystalline powders were obtained when the mol% of VDF in the copolymers was higher than 85%, while amorphous copolymers contained a lower mol% of VDF. This study also reports for the first time the determination of reactivity ratios (rVDF = 0.76 ± 0.34 and r1234yf = 1.23 ± 0.17 at 74 °C). Subsequently, iodine transfer polymerization (ITP) of VDF and 1234yf in the presence of 1-iodoperfluorohexane as the CTA in 1,1,1,3,3-pentafluorobutane and even in water using potassium persulfate as the initiator without any surfactant led to satisfactory yields (ca. 80%), Mn up to 4100 g mol−1 and a narrow Đ (ca. 1.35). A detailed kinetic study of ITP enabled assessing the chain transfer constant of C6F13I, CTr = 7.4, at 74 °C. The compositions and microstructures of all the obtained copolymers were determined by 1H and 19F NMR spectroscopies. Finally, chemical modification of the iodide end functionality of the poly(VDF-co-1234yf)–I copolymer into a primary hydroxyl end group was achieved by radical addition of these iodinated poly(VDF-co-1234yf) copolymers onto allyl alcohol, followed by selective reduction of iodine atoms.

 Please wait while we load your content...
Please wait while we load your content...