Cheap and fast: oxalic acid initiated CO2-based polyols synthesized by a novel preactivation approach†
Abstract
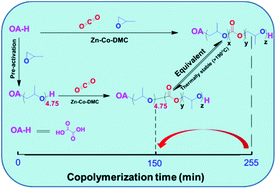
Oxalic acid, the cheapest dicarboxylic acid, was used as an effective initiator to synthesize polyols by copolymerization of CO2 and propylene oxide over a zinc–cobalt double metal cyanide catalyst. Generally, reaction times as long as 255 min were observed for complete PO conversion, due to the existence of the free carboxylic acid group of oxalic acid. To overcome this disadvantage, we proposed a novel preactivation approach by formation of oxalic acid based oligo-ether-diol in advance. About 4.75 PO monomers were initiated at 80 °C, which was independent of time and oxalic acid amount; the diol then acted as a chain transfer agent for the following copolymerization. Under the optimal conditions the reaction could proceed to completion in 150 min, which was a remarkable reduction in reaction time compared to the previous reaction time of 255 min. Notably, the resulting CO2-based diol was stable up to 190 °C, indicating that oxalic acid may be applied as an effective initiator for this copolymerization.

 Please wait while we load your content...
Please wait while we load your content...