Epimerization of C5 of an N-hydroxypyrrolidine in the synthesis of swainsonine related iminosugars†
Abstract
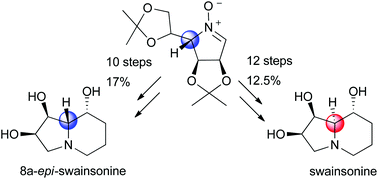
Epimerization of C5 of an N-hydroxypyrrolidine ring by regioselective oxidation to a nitrone followed by diastereoselective reduction provides a new approach to the synthesis of swainsonine and related compounds. The only protection in the synthesis of the potent mannosidase inhibitor DIM (1,4-dideoxy-1,4-imino-D-mannitol) was the acetonation of D-mannose.

 Please wait while we load your content...
Please wait while we load your content...