A surprising switch in absolute configuration of anti-inflammatory macrolactones†
Abstract
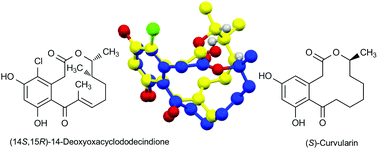
Oxacyclododecindione-type macrolactones exhibit highly potent anti-inflammatory activities even at nanomolar concentration. After the determination of the relative configuration of the stereocenters at C14 and C15 by total synthesis of 4-dechloro-14-deoxyoxacyclododecindione and 14-deoxyoxacyclododecindione, the absolute configuration has now been assigned by X-ray crystallography. Surprisingly, the absolute configuration is (14S,15R) which differs for C15 from that of the well-known derivatives of (S)-curvularin. The biological activities of both enantiomers of 14-deoxyoxacyclododecindione, obtained by racemic synthesis and optical resolution, were investigated and the ring conformation of the natural product was compared to that of (S)-curvularin and (R)-dehydrocurvularin.

 Please wait while we load your content...
Please wait while we load your content...