Total synthesis of haliclamide†
Abstract
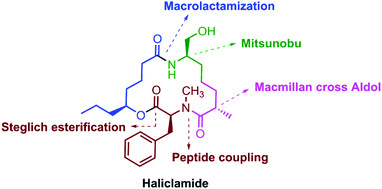
A stereoselective approach for the synthesis of haliclamide 1, a marine natural product, has been developed. The notable features of our synthesis include MacMillan cross aldol, Mitsunobu inversion, Yamaguchi–Hirao alkylation, Steglich esterification and macrolactamization reactions and the Corey–Fuchs protocol as the key steps.


 Please wait while we load your content...
Please wait while we load your content...