Total synthesis of diptoindonesin G and its analogues as selective modulators of estrogen receptors†
Abstract
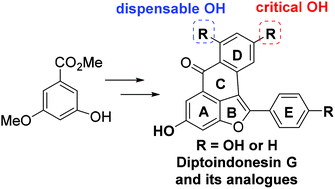
We have developed a versatile synthetic strategy for the synthesis of the natural product diptoindonesin G and its analogues as selective modulators of estrogen receptors. The strategy involves a regioselective dehydrative cyclization of arylacetals, a regioselective bromination of benzofurans, a sequential cross-coupling of bromo-benzofurans with aryl boronic acids, and a BBr3-mediated tandem cyclization and demethylation. Preliminary biological studies uncovered the critical and dispensable phenolic hydroxyl groups in the natural product and also revealed unexpected selectivity for isoforms of estrogen receptor.

 Please wait while we load your content...
Please wait while we load your content...