The Bull–James assembly as a chiral auxiliary and shift reagent in kinetic resolution of alkyne amines by the CuAAC reaction†‡
Abstract
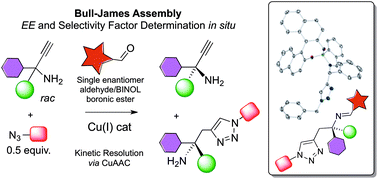
The Bull–James boronic acid assembly is used simultaneously as a chiral auxiliary for kinetic resolution and as a chiral shift reagent for in situ enantiomeric excess (ee) determination by 1H NMR spectroscopy. Chiral terminal alkyne-containing amines, and their corresponding chiral triazoles formed via CuAAC, were probed in situ. Selectivity factors of up to s = 4 were imparted and measured, accurate to within ±3% when compared to chiral GC.
- This article is part of the themed collection: Chemosensors and Molecular Logic


 Please wait while we load your content...
Please wait while we load your content...