Synthesis of α-sulfenyl monoketones via a metal-free oxidative cross dehydrogenative coupling (CDC) reaction†
Abstract
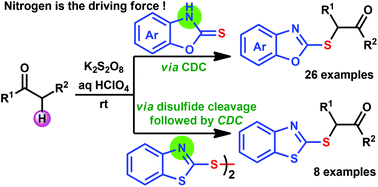
α-Sulfenyl ketones are potential precursors which find a variety of applications in organic synthesis. Their typical synthesis requires pre-functionalized starting materials and two to three step synthetic sequences. In addition, the selective pre-functionalization of unsymmetrical ketones is a challenge, which limits the synthesis of the desired sulfenylated ketones. To overcome these disadvantages, a metal-free, convenient one-step strategy for synthesizing α-sulfenyl ketones at ambient temperature via a cross-dehydrogenative coupling (CDC) strategy has been developed with a broad substrate scope. Therefore, this CDC strategy for C–S bond formation is attractive and may find wide applications in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...