Synthesis of 2,6-trans- and 3,3,6-trisubstituted tetrahydropyran-4-ones from Maitland–Japp derived 2H-dihydropyran-4-ones: a total synthesis of diospongin B†
Abstract
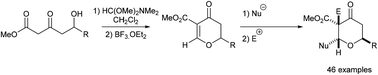
6-Substituted-2H-dihydropyran-4-one products of the Maitland–Japp reaction have been converted into tetrahydropyrans containing uncommon substitution patterns. Treatment of 6-substituted-2H-dihydropyran-4-ones with carbon nucleophiles led to the formation of tetrahydropyran rings with the 2,6-trans-stereochemical arrangement. Reaction of the same 6-substituted-2H-dihydropyran-4-ones with L-Selectride led to the formation of 3,6-disubstituted tetrahydropyran rings, while trapping of the intermediate enolate with carbon electrophiles in turn led to the formation 3,3,6-trisubstituted tetrahydropyran rings. The relative stereochemical configuration of the new substituents was controlled by the stereoelectronic preference for pseudo-axial addition of the nucleophile and trapping of the enolate from the opposite face. Application of these methods led to a synthesis of the potent anti-osteoporotic diarylheptanoid natural product diospongin B.


 Please wait while we load your content...
Please wait while we load your content...