Replacing a single atom accelerates the folding of a protein and increases its thermostability†
Abstract
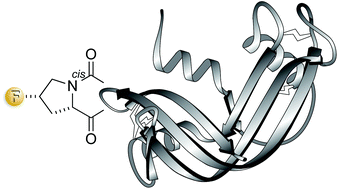
The conformational attributes of proline can have a substantial effect on the folding of polypeptide chains into a native structure and on the stability of that structure. Replacing the 4S hydrogen of a proline residue with fluorine is known to elicit stereoelectronic effects that favor a cis peptide bond. Here, semisynthesis is used to replace a cis-proline residue in ribonuclease A with (2S,4S)-4-fluoroproline. This subtle substitution accelerates the folding of the polypeptide chain into its three-dimensional structure and increases the thermostability of that structure without compromising its catalytic activity. Thus, an appropriately situated fluorine can serve as a prosthetic atom in the context of a protein.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...