Synthesis of misfolded glycoprotein dimers through native chemical ligation of a dimeric peptide thioester†
Abstract
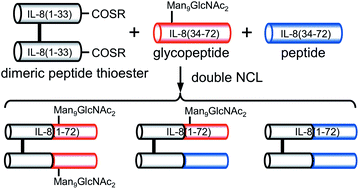
Glycoprotein quality control processes are very important for an efficient production of glycoproteins and for avoiding the accumulation of unwanted toxic species in cells. These complex processes consist of multiple enzymes and chaperones such as UGGT, calnexin/calreticulin, and glucosidase II. We designed and synthesized monomeric and dimeric misfolded glycoprotein probes. Synthetic homogeneous monomeric glycoproteins proved to be useful substrates for kinetic analyses of the folding sensor enzyme UGGT. For a concise synthesis of a bismaleimide-linked dimer, we examined double native chemical ligation (dNCL) of a dimeric peptide-α-thioester. The dNCL to two equivalents of glycopeptides gave a homodimer. The dNCL to a 1 : 1 mixture of a glycopeptide and a non-glycosylated peptide gave all the three possible ligation products consisting of two homodimers and a heterodimer. Both the homodimer bearing two Man9GlcNAc2 (M9) oligosaccharides and the heterodimer bearing one M9 oligosaccharide were found to be good substrates of UGGT.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...