Total synthesis, biosynthesis and biological profiles of clavine alkaloids
Abstract
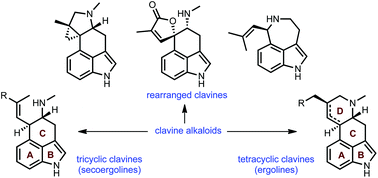
This review highlights noteworthy synthetic and biological aspects of the clavine subfamily of ergot alkaloids. Recent biosynthetic insights have laid the groundwork for a better understanding of the diverse biological pathways leading to these indole derivatives. Ergot alkaloids were among the first fungal-derived natural products identified, inspiring pharmaceutical applications in CNS disorders, migraine, infective diseases, and cancer. Pergolide, for example, is a semi-synthetic clavine alkaloid that has been used to treat Parkinson's disease. Synthetic activities have been particularly valuable to facilitate access to rare members of the Clavine family and empower medicinal chemistry research. Improved molecular target identification tools and a better understanding of signaling pathways can now be deployed to further extend the biological and medical utility of Clavine alkaloids.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...