Total synthesis of desoxycyclomarin C and the cyclomarazines A and B†
Abstract
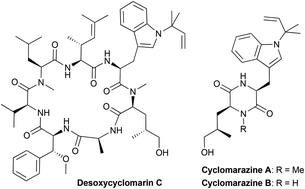
Removing the β-hydroxy group from the prenylated tryptophan moiety of cyclomarins simplifies the synthesis of these interesting natural products significantly, without having a noteworthy effect on the anti-tuberculosis activity of the cyclomarins. In contrast, cyclomarazines did not show biological activity.

 Please wait while we load your content...
Please wait while we load your content...