Towards theory driven structure elucidation of complex natural products: mandelalides and coibamide A†
Abstract
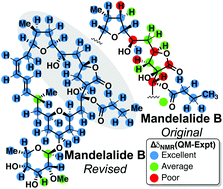
The effectiveness of computational tools in determining relative configurations of complex molecules is investigated, using natural products mandelalides A–D and coibamide A, towards a generalized recipe for the scientific community at large. Ultimately, continuing efforts in this vein will accelerate and strengthen relative structure elucidation of complex molecules, such as natural products. Molecular mechanics conformational search, quantum mechanical NMR chemical shift predictions, and DP4 analyses led to confirmation of the revised structures of mandelalides A–D and coibamide A. All chiral centers in the northern hemisphere of mandelalides A–D are inverted with respect to the originally proposed structures, in agreement with recent total syntheses of mandelalide A by Ye, Fürstner & Carter. In the case of coibamide A, it was found that Fang & Su's revision, in which both the macrocycle [MeAla11] and the side chain [HIV2] residues are inverted from L to D, was consistent with the authentic natural product and computations.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...