Taming tosyl azide: the development of a scalable continuous diazo transfer process†
Abstract
Heat and shock sensitive tosyl azide was generated and used on demand in a telescoped diazo transfer process. Small quantities of tosyl azide were accessed in a ‘one pot’ batch procedure using shelf stable, readily available reagents. For large scale diazo transfer reactions tosyl azide was generated and used in a telescoped flow process, to mitigate the risks associated with handling potentially explosive reagents on scale. The in situ formed tosyl azide was used to rapidly perform diazo transfer to a range of acceptors, including β-ketoesters, β-ketoamides, malonate esters and β-ketosulfones. An effective in-line quench of sulfonyl azides was also developed, whereby a sacrificial acceptor molecule ensured complete consumption of any residual hazardous diazo transfer reagent. The telescoped diazo transfer process with in-line quenching was used to safely prepare over 21 g of an α-diazocarbonyl in >98% purity without any column chromatography.
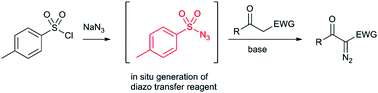

 Please wait while we load your content...
Please wait while we load your content...