Structure of olefin–imidacloprid and gas-phase fragmentation chemistry of its protonated form†
Abstract
One of the major insect metabolites of the widely used neonicotinoid insecticide imidacloprid, 1 (1-[(6-chloro-3-pyridinyl)methyl]-N-nitro-1H-imidazol-2-amine), is the olefin 2. To better understand how the structure of olefin 2 relates to the gas-phase fragmentation of its protonated form, 2H+, X-ray crystallography, tandem mass spectrometry experiments and DFT calculations were carried out. Olefin 2 was found to be in a tautomeric form where the proton is on the N(1) position of the imidazole ring and forms a hydrogen bond to one of the oxygen atoms of the coplanar nitroamine group. Under conditions of low-energy collision-induced dissociation (CID) in a linear ion trap, 2H+, formed via electrospray ionization (ESI), fragments via a major loss of water, together with minor competing losses of HNO2 and 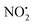 . This contrasts with 1H+, which mainly undergoes bond homolysis via
. This contrasts with 1H+, which mainly undergoes bond homolysis via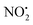 loss. Thus, installation of the double bond in 2 plays a key role in facilitating the loss of water. DFT calculations, carried out using the B3LYP/6-311G++(d,p) level of theory, revealed that loss of water was energetically more favourable compared to HNO2 and
loss. Thus, installation of the double bond in 2 plays a key role in facilitating the loss of water. DFT calculations, carried out using the B3LYP/6-311G++(d,p) level of theory, revealed that loss of water was energetically more favourable compared to HNO2 and 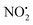 loss. Three multistep, energetically accessible mechanisms were identified for loss of water from 2H+, and these have the following barriers: (i) direct proton transfer from N(5) of the pyridine to O(1) on the NO2 group (119 kJ mol−1); (ii) rotation of the N(2)–N(4) bond (117 kJ mol−1); (iii) 1,3-intramolecular proton transfer between the two oxygen atoms of the NO2 group (145 kJ mol−1). Given that the lowest barrier for the losses of HNO2 and
loss. Three multistep, energetically accessible mechanisms were identified for loss of water from 2H+, and these have the following barriers: (i) direct proton transfer from N(5) of the pyridine to O(1) on the NO2 group (119 kJ mol−1); (ii) rotation of the N(2)–N(4) bond (117 kJ mol−1); (iii) 1,3-intramolecular proton transfer between the two oxygen atoms of the NO2 group (145 kJ mol−1). Given that the lowest barrier for the losses of HNO2 and 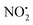 is 156 kJ mol−1, it is likely that all three water loss mechanisms occur concurrently.
is 156 kJ mol−1, it is likely that all three water loss mechanisms occur concurrently.


 Please wait while we load your content...
Please wait while we load your content...