Generation of thioethers via direct C–H functionalization with sodium benzenesulfinate as a sulfur source†
Abstract
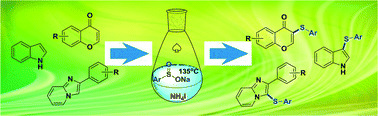
A novel ammonium iodide-induced sulfenylation method of flavones, indole and arylimidazo[1,2-a]pyridines using stable and odorless sodium benzenesulfinates as sulfur sources was developed, generating regioselective derivatives in good yields. This method has enriched current thioether-producing methods and provided a good example of using ammonium iodide as a reaction inducer instead of iodine to make thioethers under environmentally friendly and odorless conditions.

 Please wait while we load your content...
Please wait while we load your content...