Synthesis of a new β-amino acid with a 3-deoxy-l-ara furnaoside side chain: the influence of the side chain on the conformation of α/β-peptides†
Abstract
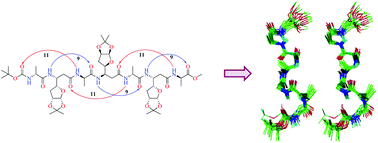
The important role of side chains in the stabilization of helical folds in peptidic foldamers containing C-linked carbo-β-amino acids (β-Caa), an interesting class of β-amino acids, with carbohydrate side chains has been extensively elaborated. As a pragmatic approach to alleviate the interference of substituents in the side chains on the folding propensities of the peptides, they are often modified or removed. The present study reports the synthesis of a new β-Caa with a 3-deoxy-L-ara furanoside side chain, [(R)-β-Caa(da)], from D-glucose, and its use in the synthesis of α/β-peptides in 1 : 1 alternation with D-Ala. The synthesis of peptides using (R)-β-Caa(da), was facile unlike those from (R)-β-Caa(a) having the L-ara furanoside side chain. The detailed NMR, molecular dynamics (MD) and CD studies on the new α/β-peptides showed the presence of robust left-handed 11/9-mixed helices. The study demonstrates that the new (R)-β-Caa(da), behaves differently compared to the other two related monomers, (R)-β-Caa(x) with the D-xylo furanoside side chain and (R)-β-Caa(a).

 Please wait while we load your content...
Please wait while we load your content...