Formation of target-specific binding sites in enzymes: solid-phase molecular imprinting of HRP†
Abstract
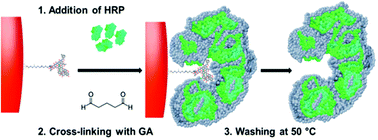
Here we introduce a new concept for synthesising molecularly imprinted nanoparticles by using proteins as macro-functional monomers. For a proof-of-concept, a model enzyme (HRP) was cross-linked using glutaraldehyde in the presence of glass beads (solid-phase) bearing immobilized templates such as vancomycin and ampicillin. The cross-linking process links together proteins and protein chains, which in the presence of templates leads to the formation of permanent target-specific recognition sites without adverse effects on the enzymatic activity. Unlike complex protein engineering approaches commonly employed to generate affinity proteins, the method proposed can be used to produce protein-based ligands in a short time period using native protein molecules. These affinity materials are potentially useful tools especially for assays since they combine the catalytic properties of enzymes (for signaling) and molecular recognition properties of antibodies. We demonstrate this concept in an ELISA-format assay where HRP imprinted with vancomycin and ampicillin replaced traditional enzyme–antibody conjugates for selective detection of templates at micromolar concentrations. This approach can potentially provide a fast alternative to raising antibodies for targets that do not require high assay sensitivities; it can also find uses as a biochemical research tool, as a possible replacement for immunoperoxidase-conjugates.

 Please wait while we load your content...
Please wait while we load your content...