Self-supported nanoporous NiCo2O4 nanowires with cobalt–nickel layered oxide nanosheets for overall water splitting†
Abstract
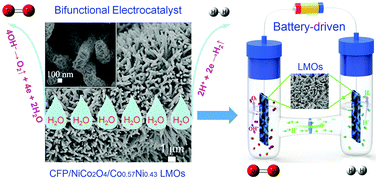
Water splitting via the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in producing H2 and O2 is a very important process in the energy field. Developing an efficient catalyst which can be applied to both HER and OER is crucial. Here, a bifunctional catalyst, CFP/NiCo2O4/Co0.57Ni0.43LMOs, has been successfully fabricated. It exhibits remarkable performance for OER in 0.1 M KOH producing a current density of 10 mA cm−2 at an overpotential of 0.34 V (1.57 V vs. RHE), better than that of the commercial Ir/C (20%) catalyst. Simultaneously, it also exhibits good catalytic performance for HER in 0.5 M H2SO4 producing a current density of 10 mA cm−2 at an overpotential of 52 mV and a Tafel slope of 34 mV dec−1, approaching that of the commercial Pt/C (20%) nanocatalyst. Particularly, CFP/NiCo2O4/Co0.57Ni0.43LMOs present better durability under harsh OER and HER cycling conditions than commercial Ir/C and Pt/C. Furthermore, an H-type electrolyzer was fabricated by applying CFP/NiCo2O4/Co0.57Ni0.43LMOs as the cathode and anode electrocatalyst, which can be driven by a single-cell battery. This bifunctional catalyst will be very promising in overall water splitting.

 Please wait while we load your content...
Please wait while we load your content...