Hydrogen bond-assisted crystallization: structure, growth and characterization of a new mixed-anion transition metal fluoride Na3NH4(TiF6)(SO4)·H2O†
Abstract
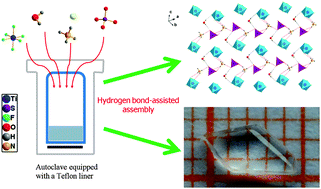
The first mixed-anion transition metal (TM) fluoride with a [MF6]x− (M = TM cation) anion group, Na3NH4(TiF6)(SO4)·H2O (NTS), was synthesized by a facile hydrothermal method. The structure of NTS features hydrogen-bonded layers constructed by four kinds of building blocks, i.e., [NH4]+, [TiF6]2−, [SO4]2−, and H2O, and four categories of hydrogen bonds, i.e., N–H⋯O, N–H⋯F, O–H⋯O and O–H⋯F, and the layers are further connected by Na+ forming a three-dimensional framework. A transparent crystal with a size up to 5.0 × 2.5 × 1.0 mm3 has been grown just by controlling the hydrothermal conditions. The crystal morphology, electronic structure, and optical and thermal properties of NTS were analyzed.

 Please wait while we load your content...
Please wait while we load your content...