New diphenyl diselenides o-substituted by an O(S,Se)-caranyl skeleton – synthesis and application in asymmetric reactions†
Abstract
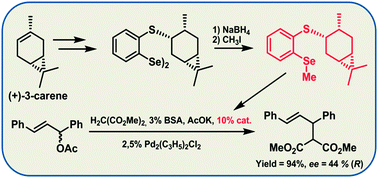
An efficient methodology for the synthesis of diphenyl diselenides o-substituted by O(S,Se)-caranyl moieties has been presented. 4-Caranyl and 4-isocaranyl groups have been connected to the phenyl ring by an oxygen, sulfur or selenium atom. The influence of the selenium–heteroatom interactions on the diastereoselectivity of the methoxyselenenylation of styrene has been evaluated. The best result was obtained for diselenide with an O-caranyl group substituted in the ortho-position. X-ray crystal structure of this compound was determined and the observed intramolecular interactions were discussed. Additionally diselenides bearing a sulfur atom were transformed to the corresponding methyl o-(S-caranyl) and o-(S-isocaranyl)-substituted phenyl selenides, and were tested as catalysts in the Tsuji–Trost allylic alkylation and Henry reaction.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...