A water molecule in the interior of a 1H-pyrazole Cu2+ metallocage†
Abstract
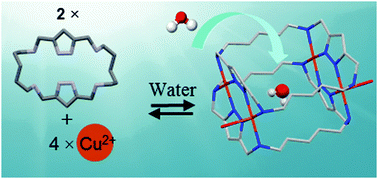
Water has a great tendency to associate through hydrogen bonding with water molecules or other hydrogen bond donor or acceptor groups. Here the case of a water molecule encapsulated in the interior of a metallocage receptor is presented. The association of four copper(II) ions and two aza-macrocyclic receptors in which two 1H-pyrazole units are connected by cadaverine diamines leads to the inclusion of a water molecule into the cage, as proved by X-ray analysis and infrared spectroscopy. The included water molecule shows no hydrogen bonding with any component of the cage presenting only a weak hydrogen bond with an oxygen atom of a perchlorate counter-anion. The IR stretching vibrations predicted by DFT calculations agree with the experimental results.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...