In silico-driven multicomponent synthesis of 4,5- and 1,5-disubstituted imidazoles as indoleamine 2,3-dioxygenase inhibitors†
Abstract
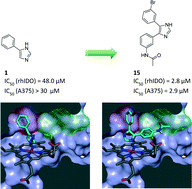
Indoleamine 2,3-dioxygenase is involved in pathological immune escape and has recently become an attractive target for anti-cancer therapy. 4-Phenylimidazole (4-PI) provides a promising starting point for the development of IDO1 inhibitors. With the aim of discovering more potent ligands, a virtual library of imidazoles synthesizable via the van Leusen multicomponent reaction was created and filtered to afford a set of 4,5- and 1,5-disubstituted imidazoles as virtual lead candidates. The compounds were selected according to their docking score and to their synthetic feasibility, synthesized and biologically evaluated. This experimental approach yielded IDO1 inhibitors with an enhanced potency compared to 4-PI; the most active compounds displayed a low micromolar potency, both in enzymatic and cellular assays, while showing no detectable cellular toxicity. A 3D quantitative structure–activity relationship based on the electrostatic and steric ligand–protein interactions was observed.

 Please wait while we load your content...
Please wait while we load your content...