Protonation state of F420H2 in the prodrug-activating deazaflavin dependent nitroreductase (Ddn) from Mycobacterium tuberculosis†
Abstract
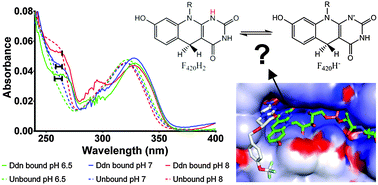
The protonation state of the deazaflavin dependent nitroreductase (Ddn) enzyme bound cofactor F420 was investigated using UV-visible spectroscopy and computational simulations. The reduced cofactor F420H2 was determined to be present in its deprotonated state in the holoenzyme form. The mechanistic implications of these findings are discussed.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...