Pathogenic mutation R959W alters recognition dynamics of dysferlin inner DysF domain
Abstract
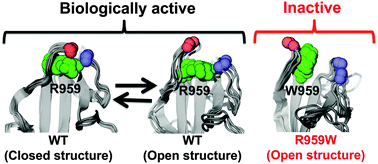
Dysferlin, a 220 kD protein, plays a major role in regulating plasma membrane repair in muscle cells. Mutations in the dysferlin inner DysF domain are known to cause different types of muscular dystrophy, including limb-girdle muscular dystrophy type 2B (LGMD2B) and Miyoshi myopathy (MM). Replacement of arginine in position 959 by tryptophan has been frequently associated with both LGMD2B and MM, but the molecular mechanisms by which this mutation alters dysferlin function remain unknown. In this study, we have used protein binding site predictions and microsecond molecular dynamics (MD) simulations to determine the effects pathogenic mutation R959W on the structural dynamics of dysferlin inner DysF domain. Analysis of 2 μs long MD trajectories revealed that mutation R959W does not induce local destabilization, unfolding or misfolding of the domain. We used a binding site predictor to discover a protein-binding site (residues T958-I966 and E1031-H1037) that resembles pincers in shape. Cartesian principal component analysis and interresidue distance distributions of the wild-type domain showed that the predicted protein-binding site undergoes a pincer motion, and populates two structural states, open and closed. We found that mutation R959W inhibits the pincer motion of the protein-binding site and completely shifts the equilibrium toward the open state. These differences in the structural dynamics of the predicted binding site suggest that mutation R959W alters recognition dynamics of the inner DysF domain. Based on these findings and on previous experimental studies, we propose a novel role for the inner DysF domain in muscle membrane repair through recruitment of dysferlin to plasma membrane. In conclusion, these findings have important implications for our understanding of the structural aspects of muscular dystrophies in atomic-level resolution.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...