A systematic molecular dynamics approach to the structural characterization of amyloid aggregation propensity of β2-microglobulin mutant D76N†
Abstract
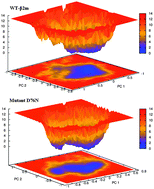
Beta-2 microglobulin (β2m) is an amyloidogenic protein belongs to the immunoglobulin superfamily, responsible for the dialysis-related amyloidosis (DRA). Misfolding of β2m is a prerequisite to the formation of systemic amyloidosis that has an effect on the structure and function of the affected organ. The aim of our present study is to intensively explore the structural characterization of amyloid aggregation propensity of recently identified natural mutation D76N by applying the classical molecular dynamics (MD) approach. The MD result revealed that mutant D76N exhibited a wide variation in the evolutionarily conserved secondary structure profile. Due to an unsatisfied position of main chain donor/acceptor atoms that unable to form essential hydrogen bonds resulted to cause misfolding of mutant D76N by disrupting the local folding of β-strands and turn region. Analysis of time evolution of various structural properties, especially those of the functionally important residues: aggregation determining, initiating, and gatekeeper residues gave some possible insights into the structural characteristics of the disease mutant D76N. In a nutshell, compared to the wild-type β2m, aggregation promoting propensity of mutant D76N has established a long β-strand D owing to an inward movement of residue, Asp53. Besides, aggregation forming characteristic of the DE loop in mutant D76N showed greater flexibility along the first principal eigenvector that favored to enhance an unusual conformational dynamics may lead toward self-aggregation and amyloid fibrils.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...