Bulk sensitive determination of the Fe3+/FeTot-ratio in minerals by Fe L2/3-edge X-ray Raman scattering
Abstract
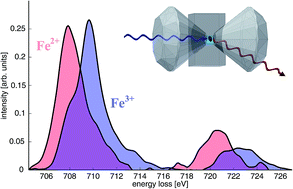
We present the first measurements of the iron L2/3-edge of the compounds FeO, Fe2O3, and Fe3O4 at ambient pressure and of FeCO3 at high pressures of 2.4 and 40 GPa using a diamond anvil cell by X-ray Raman scattering spectroscopy, a bulk sensitive probe of soft X-ray absorption edges making use of hard X-rays. We show that the spectral shape of the Fe L2/3-edge can be analyzed quantitatively to reveal the oxidation state of iron in matter. Consequently, in situ X-ray Raman scattering spectroscopy at the iron L-edge at high pressure and temperature opens exciting perspectives to characterize the local coordination, oxidation, and spin state of iron at high pressure and temperature, conditions that are of relevance for e.g. geological sciences or chemical processing.

 Please wait while we load your content...
Please wait while we load your content...