Electrode instead of catalyst and enzyme. A greener protocol for the synthesis of new 2-hydroxyacetamide derivatives containing a γ-lactone ring†
Abstract
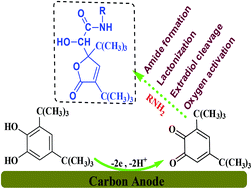
Electrochemical synthesis of some 2-hydroxyacetamides containing a γ-lactone ring was carried out by anodic oxidation of 3,5-di-tert-butylcatechol in the presence of primary aliphatic amines at the surface of the carbon electrode. This transformation was triggered by extradiol ring cleavage of electrogenerated o-benzoquinone followed by lactonization and amide bond formation leading to 2-hydroxyacetamide derivatives. In this work, some new 2-hydroxyacetamides in a single step without any enzyme, catalyst and activator in good to high yields, without toxic reagents at a carbon electrode using a sustainable method, are synthesized.

 Please wait while we load your content...
Please wait while we load your content...