Direct conversion of methanol to n-C4H10 and H2 in a dielectric barrier discharge reactor
Abstract
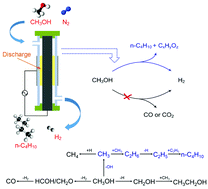
Methanol is an important H-carrier and C1 chemical feedstock. In this paper, a direct conversion of methanol to n-C4H10 and H2 was achieved for the first time in a dielectric barrier discharge (DBD) non-thermal plasma reactor. The selective formation of n-C4H10 by limiting COx (x = 1 and 2) generation was obtained by optimizing different plasma processing parameters including the methanol inlet concentration, discharge power, and pre-heating temperature. The results showed that a higher methanol inlet concentration and a higher pre-heating temperature favors the formation of n-C4H10, while a higher methanol inlet concentration and a lower discharge power can effectively limit the formation of CO. The optimal selectivity for n-C4H10 (37.5%), H2 (28.9%) and CO (14%) was achieved, with a methanol conversion of 40.0%, at a methanol inlet concentration of 18 mol%, a discharge power of 30 W and a pre-heating temperature of 140 °C using N2 as a carrier gas. Value-added liquid chemicals (e.g., alcohols, acids, and heavy hydrocarbons) were also obtained from this reaction. Emission spectroscopy diagnostics reveals the formation of various reactive species (e.g., CH, C2, CN, H and metastable N2) in the CH3OH/N2 DBD. Possible reaction pathways for the formation of n-C4H10 were proposed and discussed.

 Please wait while we load your content...
Please wait while we load your content...