Metal-free, visible-light-mediated, decarboxylative alkylation of biomass-derived compounds†
Abstract
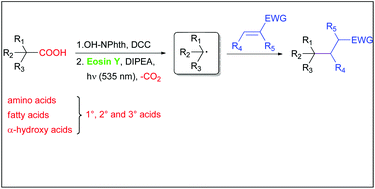
This work describes a mild, environmentally friendly method to activate natural carboxylic acids for decarboxylative alkylation. After esterification of biomass-derived acids to N-(acyloxy)phthalimides, the active esters are cleaved reductively by photocatalysis to give alkyl radicals, which undergo C–C bond formation with electron-deficient alkenes. This reaction is catalyzed by the organic dye eosin Y and green light (535 nm) and the scope of acids includes abundant amino acids, α-oxy acids and fatty acids which are available from renewable resources.


 Please wait while we load your content...
Please wait while we load your content...