Chemoselective hydrogenation of 3-nitrostyrene over a Pt/FeOx pseudo-single-atom-catalyst in CO2-expanded liquids†
Abstract
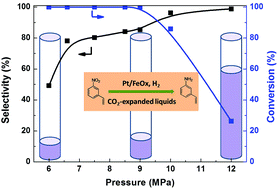
Chemoselective hydrogenation of substituted nitroarenes containing two reducible groups in one molecule is a highly desired approach to the synthesis of functionalized anilines. To make this process environmentally benign, we used a pseudo-single-atom-catalyst Pt/FeOx and investigated the reaction in supercritical CO2 and CO2-expanded toluene. The results showed that supercritical CO2 afforded excellent selectivity but low reactivity due to the limited substrate solubility in the reaction medium. By contrast, when the reaction proceeded in CO2 expanded toluene, both the conversion of 3-nitrostyrene and the selectivity of 3-vinylaniline reached above 95% under optimum conditions while the organic toluene amount could be reduced by 90% compared to that without CO2. The thermodynamic calculations revealed that the solubility of H2 increased while the viscosity of the reaction system decreased with the CO2 pressure, which facilitated the mass transfer and therefore increased the reaction rate meanwhile keeping the selectivity at a high level.

 Please wait while we load your content...
Please wait while we load your content...